Explain Why Sublimation and Deposition Are Classified as Physical Changes
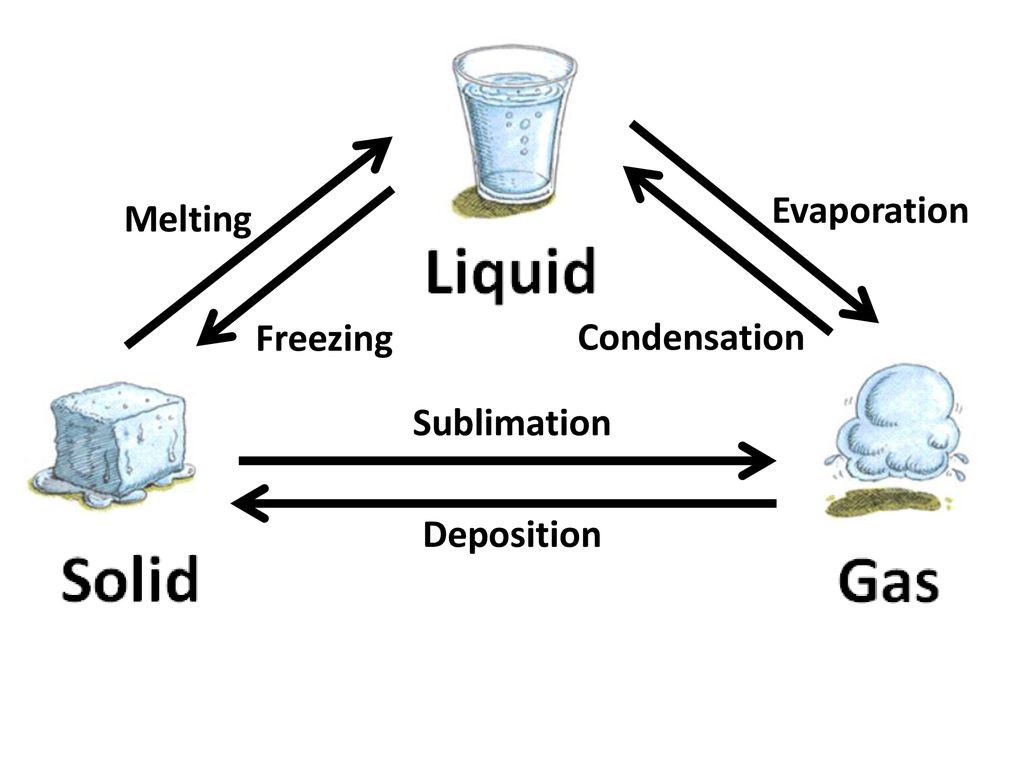
Phase changes include vaporization condensation melting freezing sublimation and deposition. Evaporation a type of vaporization occurs when particles of a liquid reach a high enough energy to leave the surface of the liquid and change into the gas state.
Kaupapa 25 05 Thursday Define Sublimation Ppt Download
These processes do not have any effect on the chemical composition or properties of the substances.

. Chemical changes involve changes in chemical composition and require chemical reactions. So sublimation when a substance goes directly from a solid to a gas and deposition just the opposite is just a changing of the physical state of a substance just like something melting or freezing while changes such as burning turn the substance being burned into something totally new making it a chemical change. A physical change is a type of change of matter that is not dependent upon electron behavior unlike chemical change.
The material moved by erosion is sediment. Deposition- rust frost Sublimation- snow on a sunny day dry ice. That is sublimation is a kind of physical change.
Deposition occurs when the agents wind or water of erosion lay down sediment. Infiltration of opal templates with catalytic material has been achieved by various methods and can be broadly classified into solution-based growth methods and physical deposition methods. At room temperature table salt is a solid and acetone is a.
Explain why sublimation and deposition are classified as physical changes. How can the mass of a pile of snow decrease on a sunny day when the air temperature does not rise above 0 degrees C. A substances identity does not change during sublimation or deposition.
Explain why sublimation and deposition are classified as physical changes. They are the same matter but in different phases. Explain why sublimation and deposition are classified as physical changes.
Explain why sublimation and deposition are classified as physical changes. Explain why sublimation and deposition are classified as physical changes. YOU MIGHT ALSO LIKE.
Explain why an aerosol can should never be thrown into a fireplace or incinerator. Sublimation and deposition are classified as physical changes because both these processes only affect the arrangement of atoms inside the substance. Changes of state are physical changes.
FlexBook Platform CK-12 Overview. An example of evaporation is a puddle of water drying out. Explain why sublimation and deposition are classified as physical changes.
Deposition changes the shape of the land. One example of deposition is the process by which in sub-freezing air water vapour changes directly to ice without first becoming a liquidThis is how frost and hoar frost form on the ground or other surfaces. The substance changes directly from a.
Waters movements both on land and underground cause weathering and erosion which change the lands surface features and create underground formations. Explain Why Changes Of State Are Physical Changes. Sublimation is a change in the physical state from liquid phase to gas phase that does not involve an intermediate liquid phase commonly observed at habitable temperatures and pressures.
Processes in which matter changes between liquid and solid states are freezing and melting. Changes in matter can be classified as either physical or chemical like matter properties. What causes frost to form on windows.
Physical changes include changes in physical appearance but not composition. Explain why an aerosol can should never be thrown into a fireplace or incinerator. The substance changes directly from a solid to a gas without going through the liquid phase.
Explain why there. Sublimation and deposition are classified as physical changes because both these processes only affect the arrangement of atoms inside the substance. Processes in which matter changes between liquid and gaseous states are vaporization evaporation and condensation.
All changes in state of matter are physical changes. Sublimation and deposition are physical changes because you havent changed the chemical nature of the substance. Another example is when frost forms on a leaf.
Explain why sublimation and deposition are classified as physical changes. Solution-growth methods include solutiongelation solgel chemistry dip coating spin coating and electrochemical deposition while physical deposition methods include atomic layer. They occur when matter absorbs or loses energy.
For deposition to occur thermal energy must be removed from a gas. Give an example of deposition. A substances identity does not change during sublimation or deposition.
These processes do not have any effect on the chemical composition or properties of the substances.
Sublimation And Deposition Energy Education

Solved Deposition Is The Opposite Of Sublimation It Is Physical Change In Which The Substance Transitions From Its Gaseous State Directly To Its Solid State Is This Process Endothermic Or Exothermic Why

Difference Between Sublimation And Deposition Compare The Difference Between Similar Terms
No comments for "Explain Why Sublimation and Deposition Are Classified as Physical Changes"
Post a Comment